 Doctors prescribe type-2 diabetes treatment Januvia as an effective solution for lowering blood-sugar levels. Also known as sitagliptin, this diabetic medication manufactured by Merck Pharmaceuticals has taken center stage for its life-threatening side effects. After several FDA warnings and alerts, research leads experts to believe that Januvia and similar medications may encourage the development certain carcinomas (cancers) and pancreatitis (inflammation of the pancreas).
Doctors prescribe type-2 diabetes treatment Januvia as an effective solution for lowering blood-sugar levels. Also known as sitagliptin, this diabetic medication manufactured by Merck Pharmaceuticals has taken center stage for its life-threatening side effects. After several FDA warnings and alerts, research leads experts to believe that Januvia and similar medications may encourage the development certain carcinomas (cancers) and pancreatitis (inflammation of the pancreas).
Since the U.S. Food and Drug Administration approved Januvia in 2006, this new medicine has not undergone sufficient trials to confirm a correlation between many of the adverse events and the type-2 diabetes drug. However, recent findings support the concerns regarding Januvia side effects and extreme reactions such as pancreatitis, pancreatic cancer and thyroid cancer.
Dipeptidyl peptidase (DPP)-4 enzymes are known to control and inhibit the spread of cancerous cells. According to health professionals, Glucagon-like peptide-1-based therapies for type-2 diabetes such as sitagliptin (Januvia) and exenatide (Byetta) block DPP-4 enzymes in the body to raise insulin production. Consequently, the medication lowers the body’s defense system against cancers. Because of this action of type-2 diabetes medicines, the U.S. Food and Drug Administration and other researchers have gone forth to assess the potential for this threat.
Data released in 2011 by the University of California illustrates that persons treated with sitagliptin were three times more likely to develop cancer of the pancreas than those who had not been treated with the medication. Research conducted by the Gastroenterological Association claims that treatments with sitagliptin had raised the risk of pancreatic cancer with the odds being 6.7 when compared to other type-2 diabetes medications. These lethal side effects have left several dead after suffering from the harmful side effects of pancreatic cancer.
Since Januvia impacts the body’s immune system, it also increases the likelihood for developing thyroidal carcinoma as well. Furthermore, since Januvia and other glucagon-like peptide-1-based medications reduce the body’s ability to fight cancers, the carcinoma may spread throughout the body. Although thyroidcancer is rare, it can be devastating and even fatal.
If the risks of pancreatic and thyroid cancer associated with Januvia weren’t enough, further investigations link this diabetes drug to pancreatitis as well. From October 2006 to February 2009 the U.S. Food and 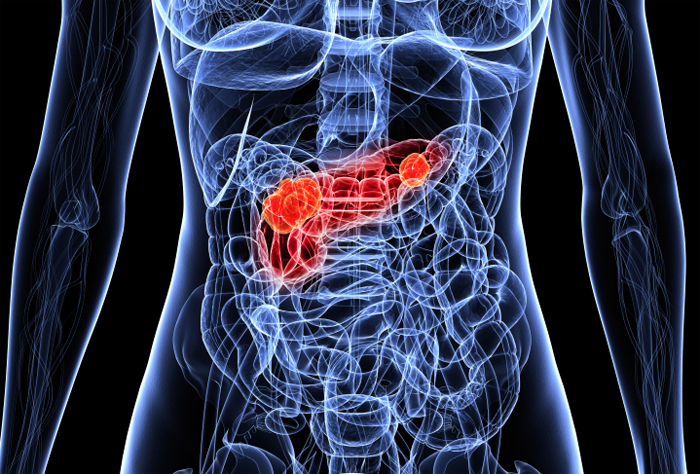 Drug Administration was notified of 88 cases of acute pancreatitis related to the use of Januvia. Research demonstrates a six-fold increase for the risk of pancreatitis in patients taking Januvia or Byetta. This includes hemorrhagic pancreatitis, a severe inflammation of the pancreas causing a hemorrhage or bleeding, and necrotizing pancreatitis, a condition in which the pancreas begins to attack itself and pancreatic tissue is destroyed.
Drug Administration was notified of 88 cases of acute pancreatitis related to the use of Januvia. Research demonstrates a six-fold increase for the risk of pancreatitis in patients taking Januvia or Byetta. This includes hemorrhagic pancreatitis, a severe inflammation of the pancreas causing a hemorrhage or bleeding, and necrotizing pancreatitis, a condition in which the pancreas begins to attack itself and pancreatic tissue is destroyed.
Persons who have been injured or suffered from adverse reactions related to Januvia may be entitled to compensation. If you or a loved one has become a victim of Januvia or related drugs, get assistance through our expert attorneys by completing the “Request Attorney Assistance” form located at the top, right-hand section of this page.
Sources: USA Today Studies
Drug Risk- Prescription Drug Patient Resource Center
0."/>