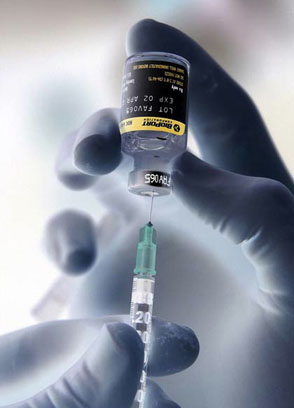
 Last year on March 29, 2012, Hospira Inc. notified consumers of a voluntary nationwide recall regarding sodium chloride injections containing particulates. According to previous reports, the medications had been contaminated by brass particles which had come in contact with the injection solutions. On April 26, 2013, administration at Hospira released an update of this recall to ensure proper contact information for Stericycle.
Last year on March 29, 2012, Hospira Inc. notified consumers of a voluntary nationwide recall regarding sodium chloride injections containing particulates. According to previous reports, the medications had been contaminated by brass particles which had come in contact with the injection solutions. On April 26, 2013, administration at Hospira released an update of this recall to ensure proper contact information for Stericycle.
As a reminder, the recall involves 0.9% sodium chloride injections which may be identified by:
For further information about the prior recall notification, click here.
The newly revised recall urges patients or healthcare professionals with recalled 0.9% sodium chloride injections to terminate use and close off the product. Consumers with recalled products should contact Stericycle by calling 1-877-597-9582 in order to properly return the recalled injections. Patients may receive replacement products from unaffected lots if needed.
Find out more about recent recalls and updates at bad-drug.net!
0."/>