Drugs seeking approval by the FDA must first undergo research by the Center for Drug Evaluation and Research (CDER) for quality, safety and effectiveness.Manufacturers must first find the need and benefit of their drug through rigorous testing, and submit these laboratory results to the CDER in the form of an Investigational New Drug (IND). The testing that the company will undertake are lab tests and detailed animal testing, all to ensure that human clinical trials will be as harmless as possible.
After a leniency period of 30 days, which will give the FDA sufficient time to look ov
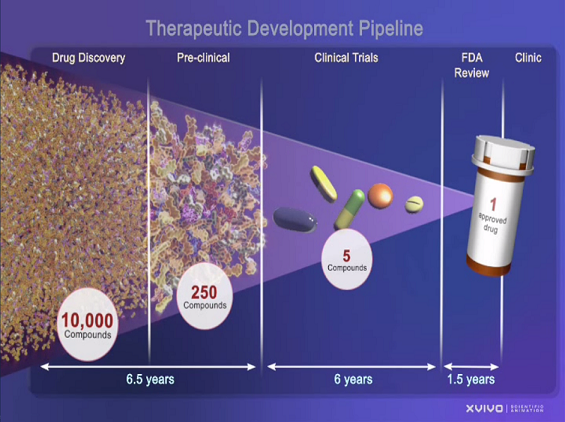
The testing before a drug reaches the FDA review process can reduce the amount of harmful drugs on the market.
er the submitted IND, the company can begin a clinical trial. Should the FDA encounter a problem with the study, they can place it on a “clinical hold.” These clinical trials normally follow 3 phases used to determine if a drug is effective, and to determine the adverse effects.
Phase I involves 20-80 people and starts collecting information on the safety of the ranges of dosages administered and records side effects. Phase II involves several hundred people to determine the efficacy of the drug, and continue dosage, side effect and safety recordings. Phase III involves several thousand subjects to reaffirm the learnings of phase II, and to closely monitor the drug when compared to its competitor.
All this information is gathered together to determine if the drug meets all the FDA’s requirements for marketing approval. After all of the clinical trials are complete, the company will submit a New Drug Applicati
on (NDA) which will include the test results, manufacturing information and specifications (including data availability, analysis of dosages, toxicology studies) to showcase that the company will responsibly market the drug, and the packaging labeling which will provide important information about the drug (efficacy and risks).The FDA will review the NDA and either approve it based on the benefits and on the idea that the company has successfully shown that it will manufacture the product with quality and respon
sibility and can now be marketed, or the FDA will mandate more testing to meet its requirements.Even if the drug has been approved by the FDA, the human clinical trials will continue after the product has been taken to market. These trials will determine the best use of the drug, new uses, new risks, and new information in determining prescribing dosages, which will all help physicians and patients determine if the drug suits a particular situation.
Before drugs are submitted in an IND they are compounds that are “in the pipeline,” with the name being only a series of letters and numbers. Once the drug is ready for the FDA reviewing process, it will receive a br
and name for submission. Drugs will be protected under patent during development and for a limited time after the drug reaches the market, giving that company exclusivity on drug sales. After the patent has expired, other companies can apply/request to sell the generic form of that brand name drug.
0."/>