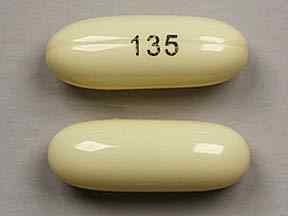
Nimodipine comes in an opaque, light yellow capsule with 135 written in black on the back side (as shown above).
As of September 4, 2012, Sun Pharmaceuticals Industries, Inc. has issued a recall for the brain hemorrhage treatment, Nimodipine. This recall was made voluntarily by the manufacturer due to a crystallization of the medicine within the capsules of Nimodipine which may have occurred during manufacturing. According to the U.S. Food and Drug Administration, the recall is a precaution to avoid potential harm that this substance could cause for patients.
Nimodipine is a calcium channel blocker medicine, generally prescribed to patients who have had a rupturing of a blood vessel in the brain, also known as a brain hemorrhage. This medicine comes in capsule, intravaneous injection and liquid form, but the affected product is the Nimodipine 30 mg capsule which of a pale yellow, oblong capsule. The recall involves certain lots of Nimodipine in blister packs of 100 or 30 capsules.
If you or someone you know uses Nimodipine 30 mg and has purchased this medicine between June 19 and April 24 of 2012, check to make sure the product has not been affected. Lot numbers recalled include lot 3305.039A and B. If you possess Nimodipine 30 mg that has been recalled, end use of the drug immediately and contact Inmar Inc. by calling 1-800-967-5952.
No serious reactions have been reported during the recall. However, under the circumstance of developing an adverse reaction which may have been caused by taking Nimodipine that has been recalled, contact the FDA’s MedWatch Adverse Event Reporting program. Patients may do so by filing a report online at www.fda.gov/medwatch/report.htm, via mail (see FDA online), or fax by the number 1-800-FDA-0178.
Read more at bad-drug.net for up-to-date medical news, recalls, and information.
0."/>