Warnings & Recalls for Introvale
Introvale Recall: Packaging Error
On
June 6, 2012, the FDA released a
safety warning concerning an
error in the packaging of Introvale.
According to
Sandoz, a producer of the contraceptive, between
January 2011 and
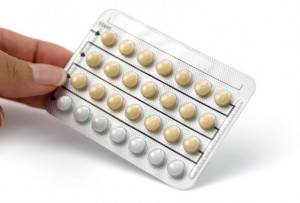
May 2012 there was a mix-up in some blister card packets of Introvale produced on the lots. The 9th row and the 13th row of pills on the the card were switched, placing the white
placebo pills in the 9th row instead of the 13th where, instead, there were 7
active peach-colored tablets. Sandoz ordered a
voluntary recall for 10 lots of Introvale.
Recalled lots of Introvale include: LF00478C, LF00479C, LF00551C, LF00552C, LF00687C, LF00688C, LF00763C, LF00764C, LF00765C and LF01261C.
What if I Have a Box of Introvale Containing an Error?
If you notice that you have taken Introvale from a box containing the error, Sandoz recommends that you
immediately begin taking a
non-hormonal form of contraception and call their doctor. You may also
report the problem at
Sandoz Drug Information Direct Line at (800)525-2492 or by emailing
[email protected].
If you are taking Introvale, it is important to
carefully read the instructions to avoid this issue.
Warnings Regarding Introvale
Women who are taking Introvale
should not smoke or use tobacco, as this may
increase the possibility of
severe cardiovascular side effects.
Before taking Introvale tell your doctor if you:
- Are taking any other drugs, vitamins, supplements or medicines
- Have a history of blood clotting disorders or high blood pressure
- Have diabetes
- Have heart disease
- Have liver issues
- Are pregnant or planning on becoming pregnant
- Plan to have surgery or have had recent surgery
Introvale Treatment and Use
Introvale | levonorgestrel and ethinyl estradiol is a contraceptive tablet marketed by Novartis for the prevention of pregnancy. Introvale may also be used for the following:
- Creating a more regular period for a woman
- Reducing the amount of blood released during a period
- Decreasing pain and aching caused during menstruation
- Decreasing the possibility of developing ovarian cysts
Introvale does not prevent the transmission of sexually transmitted diseases (STDS).
How Does Introvale Work?
Introvale prevents pregnancy through several different ways. Introvale prevents the body from releasing an egg during a woman’s menstrual cycle. Introvale also alters the lining of the uterus in order to make vaginal fluids thicker, thus prohibiting sperm to attach to the walls of the uterus. Through these steps, the possibility of pregnancy while taking Introvale becomes extremely unlikely.
How Should Introvale be Taken?
For this medication to work properly, Introvale must be taken exactly as instructed by your doctor. Introvale comes in tablet form and dosage is not dependent on age, weight or other factors.
- Patients should begin taking Introvale on the first Sunday after their period
- To ensure the prevention of pregnancy, for the first week do not have unprotected sex
- After taking the first pill, continue by taking one tablet each day at the same time. Pills should be taken in order starting from the beginning of the package
- Each blister card contains pills for 91 days (13 weeks) including 84 tablets with active ingredients and the final 7 containing placebo pills to allow your body to menstruate
- Immediately after ending the blister card, continue with another packet with the following day
- In order to keep from allowing any days between packets, make sure you have an extra blister card ready to use
 which may lead to a stroke or heart attack with symptoms such as: feeling disoriented, coughing up blood, aching of the groin or calf, pain in the chest, jaw or left arm, numbness, feeling week on one side of the body, and slurred speaking
which may lead to a stroke or heart attack with symptoms such as: feeling disoriented, coughing up blood, aching of the groin or calf, pain in the chest, jaw or left arm, numbness, feeling week on one side of the body, and slurred speaking